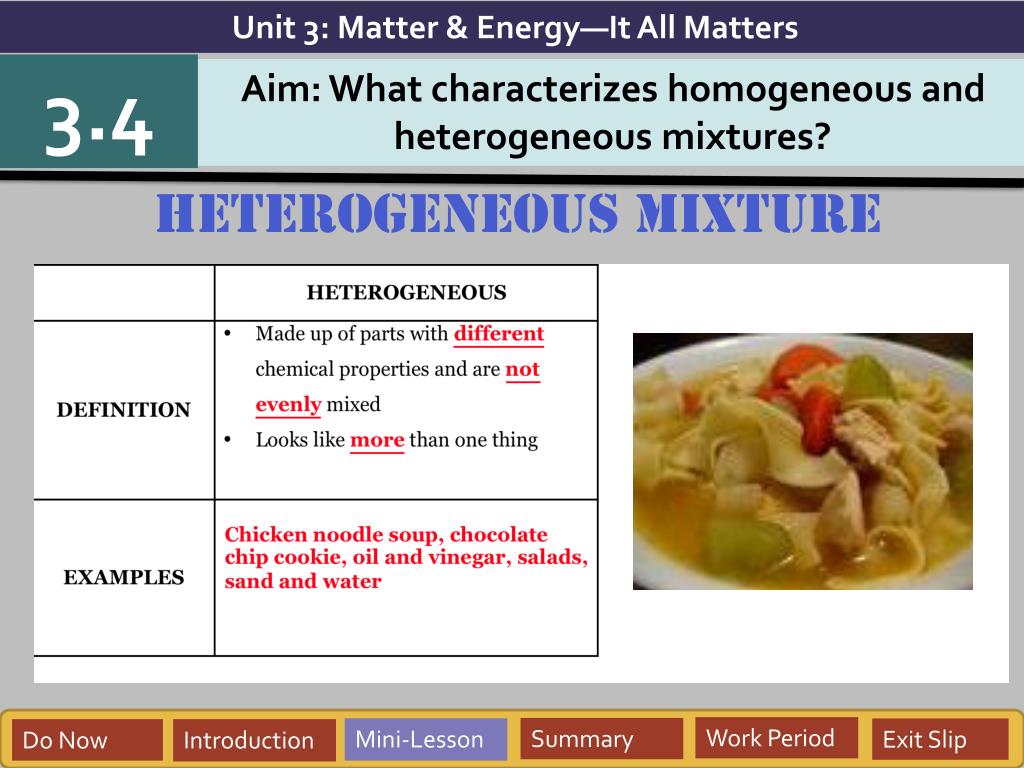
Homogeneous vs Heterogeneous Mixtures Mixtures can be either heterogeneous or homogeneous Unlike a heterogeneous mixture, a homogeneous mixture is a mixture that is uniform throughout;Heterogeneous Mixture definition Heterogeneous mixture is a mixture with a nonuniform composition When you mix two components that remain separate from each other, that mixture is called a Heterogeneous mixture Examples of Heterogeneous mixture Concrete is an example of a Heterogenous mixture A mixture of Cement and Water"Hetero" is a Greek word that means "different," "genus" means "kind" In a heterogeneous mixture, substances are NOT evenly mixed The parts of these mixtures are noticeably different from one another In simple words, If you can see the different substances in

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets
Meaning of homogeneous and heterogeneous mixture
Meaning of homogeneous and heterogeneous mixture- Definition of the heterogeneous mixture Heterogeneous mixture is the one, in which the components are not uniformly distributed throughout its volume and can be easily seen separately We can easily identify various components of a heterogeneous mixture by naked eyes or under a microscope Examples of the heterogeneous mixture –A chemical mixture combines two substances that maintain their own properties when combined Heterogeneous mixtures are made up of a nonuniform composition, while homogeneous mixtures are made up of a uniform composition For example, water and sand is a heterogeneous mixture — you can easily separate the sand from the water



Homogeneous And Heterogeneous Mixture Nine Science
Mixtures which do not have uniform composition throughout are called Heterogeneous Mixture For example – mixture of soil and sand, mixture of sulphur and iron fillings, mixture of oil and water etc The boundaries of constituent particles of a homogeneous mixture can be identified easily; Homogenous Mixture Definition A homogenous mixture is the type of mixture in which the composition of the solute is uniform throughout the mixture The homogenous mixture can be defined by the fact that the division of the mixture into two equal halves results in the distribution of the same amount of solute suspended in both of the halves The purpose of this lesson is to define what a heterogeneous mixture is and show you how to identify one We'll also list some relevant examples of heterogeneous mixtures Updated
Chemistry, 0810, vishal48 Definition of homogeneous mixture and heterogeneous mixture A homogeneous mixture is a union of two or more substances that form a joint material , in which the two original elements are indifferent, even if they are not chemically boundmore easily the elements that make up the homogenous mixture cannot be differentiated with the naked eye, but they are physically separable, since there is no chemical reactionHeterogeneous mixtures are defined as the mixture where components are mixed nonuniformly Irrespective of homogeneous mixture where components are in single phase, in heterogeneous mixture components are present in at least two different phases
Homogeneous Mixture Heterogeneous Mixture; Here, a homogeneous mixture is one in which all components are in a single phase, while a heterogeneous mixture contains components in different phases Examples of Heterogeneous Mixtures Concrete is a heterogeneous mixture of an aggregate cement, and water Sugar and sand form a heterogeneous mixture The terms homo and hetero depict the most prominent difference between homogeneous and heterogeneous mixtures The prefix homo refers to the uniformity whereas hetero indicates nonuniformity Homogeneous mixtures have a uniform composition throughout the system, and heterogeneous mixtures are the opposite



Ppt Objective I Will Distinguish Between Homogeneous And Heterogeneous Mixtures Powerpoint Presentation Id




Lesson Explainer Mixtures Nagwa
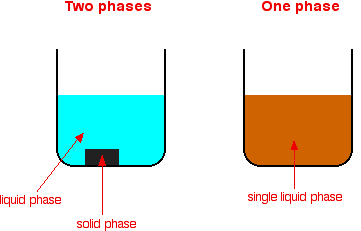
Homogeneous and heterogeneous mixtures In chemistry, if the volume of a homogeneous suspension is divided in half, the same amount of material is suspended in both halves of the substance An example of a homogeneous mixture is air In physical chemistry and materials science this refers to substances and mixtures which are in a single phaseBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phaseTypes of Mixtures A mixture is a combination of two or more substances mixing together without any chemical reaction as a result, their molecular structure does not change being not involving any chemical reaction, the substances can be separated easily at any time there are two Types of Mixtures ie, Homogeneous Mixtures and Heterogeneous mixtures




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets



Heterogeneous And Homogeneous Mixture Differences Videos Examples
Homogeneous mixture is a term in physical chemistry and material science that refers to substances and mixtures which are in a single phase This is in contrast to aWhat is the definition of a homogeneous mixture? A mixture of black pepper and salt would become a solidsolid heterogeneous mixture, where both components are distinguished by the difference in their colors Types of heterogeneous mixtures Like homogeneous mixtures, phases define the types of heterogeneous mixtures that exist




Homogeneous Mixture Definition Examples Tutors Com



Heterogeneous Mixtures
Definition of Homogeneous and Heterogeneous MixturesBackground Music CreditAlan Walker Fade NCS ReleaseVideo link https//youtube/bM7SZ5SBzyYHeterogeneous Mixture definition Heterogeneous mixture is a mixture with a nonuniform composition When you mix two components that remain separate from each other, that mixture is called a Heterogeneous mixture Examples of Heterogeneous mixture Concrete is an example of a Heterogenous mixture A mixture of Cement and Water A pure substance is a form of matter that has a fixed chemical composition and a distinct characteristic while a homogeneous mixture is a mixture of two or more compounds with compositions that are uniform or mixed together in such a way that they are indistinguishable from each other Is gold alloy homogeneous or heterogeneous?



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube
In this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference betweenA mixture is a combination of two or more substances that are not chemically united to each other Mixtures can be classified into two types homogeneous mixture and heterogeneous mixture A homogeneous mixture is one whose composition is uniform throughout the mixtureNishantietech2756 nishantietech2756 Chemistry Secondary School answered What is homogeneous mixture and heterogeneous mixture 2




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Heterogeneous And Homogeneous Mixture Differences Videos Examples
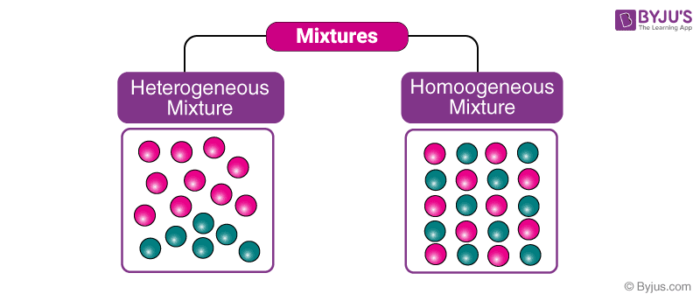
Definition of Heterogeneous Mixtures A mixture is a combination of two or more pure substances in which the original substances retain In homogeneous mixtures, the entire mixture has a uniform composition Heterogeneous mixtures have a mixed composition that can vary from point to point The components are not visible to the naked eye The components can be seen easily and distinguished The entire mixture has phase separationHomogeneous Mixture Definition A homogeneous mixture is a mixture of substances blended so thoroughly that you cannot see individual substances Every sample of the mixture will show the same amounts of each substance Homogeneous mixtures can be



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




1 2 The Classification Of Matter The Basics Of General Organic And Biological Chemistry
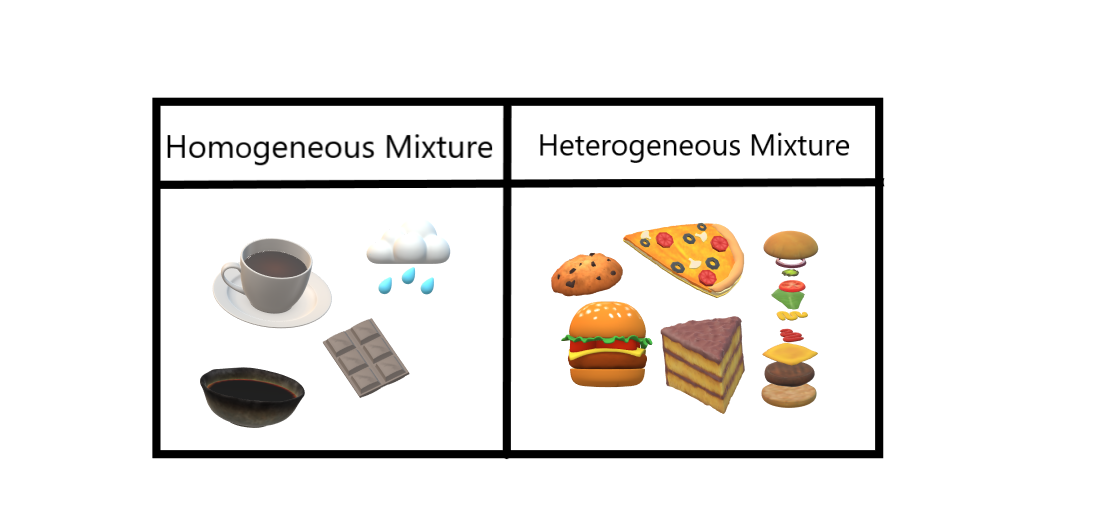
In a heterogeneous mixture the substances are not evenly distributed (chocolate chip cookies, pizza, rocks) Within the categories of homogeneous and heterogeneous mixtures there are more specific types of mixtures including solutions, alloys, suspensions, and colloids Solutions (homogeneous)Homogeneous mixture synonyms, Homogeneous mixture pronunciation, Homogeneous mixture translation, English dictionary definition of Homogeneous mixture n 1 a The act or process of mixing an alloy made from the mixture of two metals b hodgepodge A heterogeneous mixture, a jumble, a farrago, Example Mixture of Salt and Iron Filings Note Heterogeneous and homogeneous mixture may be a Matter of Scale It means that a mixture may look homogeneous from a distance But if we look closely with help of a microscope, it may appear to be heterogeneous mixture (We may be able to identify individual component clearly)



Homogeneous And Heterogeneous Mixtures Geeksforgeeks
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures
A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials Stay tuned with BYJU'S to learn more interesting topics in ChemistryThis means that the different components of the mixture cannot be distinguished from one another in some wayStart studying Heterogeneous or Homogeneous Mixture Learn vocabulary, terms, and more with flashcards, games, and other study tools



Homogeneous Vs Heterogeneous Mixture




What Is The Difference Between Heterogeneous Mixture Vs Homogenous Mixture Brainly Com
1 Mixtures that have uniform composition Mixtures that do not have uniform composition throughout 2 Boundary of separation could not be seen Boundary of separation of constituent particles is clearly visible 3 Particles are not indistinguishable Particles can be physically distinguishable 4 Homogeneous and heterogeneous are actually complete opposite of each other Homogeneous mixtures refer to a solution that mixes to the point that it becomes hard to distinguish each separate component However, in a heterogeneous mixture, the components do not mix together but instead are just in the same containerMixture, whether they are homogeneous or heterogeneous How would you separate mixtures?




Homogeneous Mixture




Chemistry For Kids Chemical Mixtures
Mixtures Homogeneous, Heterogeneous I First Impressions (≈ 1 minute) Ensures the line is silent, greet students at the door, shake their hand "Every day, this is how you will come in line up silently by the door, shake my hand, and find your seat silently These will be your assigned seats, unless otherwise told soAs a homogeneous mixture has two or more distinct phasesHomogeneous mixtures are also called as solutions Uniform composition Example rainwater, vinegar etc Heterogeneous mixture This is a type of mixture in which all the components are completely mixed and all the particles can be seen under a microscope We can easily identify the components and more than one phase can be seen by naked eyes




Q2 Differentiate Between Homog Lido




Homogeneous Mixture Examples Found At Home
A homogeneous mixture is a solid, liquid, or gaseous mixture that has the same proportions of its components throughout any given sample Conversely, a heteroge jangaiahgoudch1446 jangaiahgoudch1446 Chemistry Secondary School answered Definition of homogeneous and heterogeneous mixture 2 SeeDefinition of Homogeneous and Heterogeneous Mixture MerryCloud9 MerryCloud9 Science Elementary School answered Definition of Homogeneous and Heterogeneous Mixture 2 See answers What is homogeneous mixture and heterogeneous mixture Get the answers you need, now!




Examples Of Homogeneous Mixtures Solid Liquid And Gas



1
There are two types of mixtures heterogeneous and homogeneous Heterogeneous mixtures have visually distinguishable components, while homogeneous mixtures appear uniform throughout The most common type of homogenous mixture is a solution, which can be a solid, liquid, or gas What has to be true about available resources for competition to Homogeneous Mixture vs Heterogeneous Mixture – Types of Solutions An unadulterated substance can be a component, or an intensify that are artificially homogenous in creation and can't be isolated by any physical methods A few instances of an unadulterated substance would be Iron Metal (Fe), Salt (NaCl) and so forthHomogeneous Reaction and Heterogeneous Reaction Homogeneous Reaction Any class of reaction that occurs in a singlephase be that solid, liquid or gaseous phase is said to be a homogeneous reaction Now the homogeneous reaction may occur in a single reaction or multiple reactions can take place




Homogeneous Or Heterogeneous Mixtures Practice Worksheet




Homogeneous Mixture Definition Examples Tutors Com
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture All solutions would be considered homogeneous because the dissolved material is present in the same amount throughout the solution One characteristic of mixtures is that they can be separated into their components Homogenous vs Heterogeneous As mentioned earlier, a mixture refers to the physical coming together of substances (which, in chemistry, can be elements or compounds) There are two types of mixture (1) homogenous mixture and (2) heterogeneous mixture Heterogenous (variant heterogeneous) is the opposite of homogenousThis chemistry video tutorial explains the difference between homogeneous and heterogeneous mixtures within the subtopic of the classification of matter It
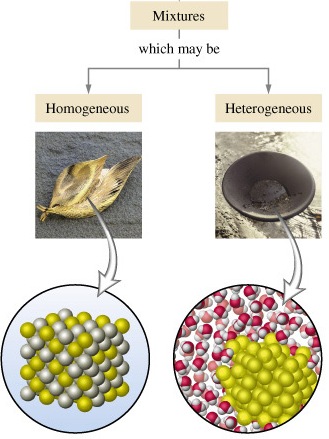



Difference Between Homogeneous Mixture And Heterogeneous Mixture




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com
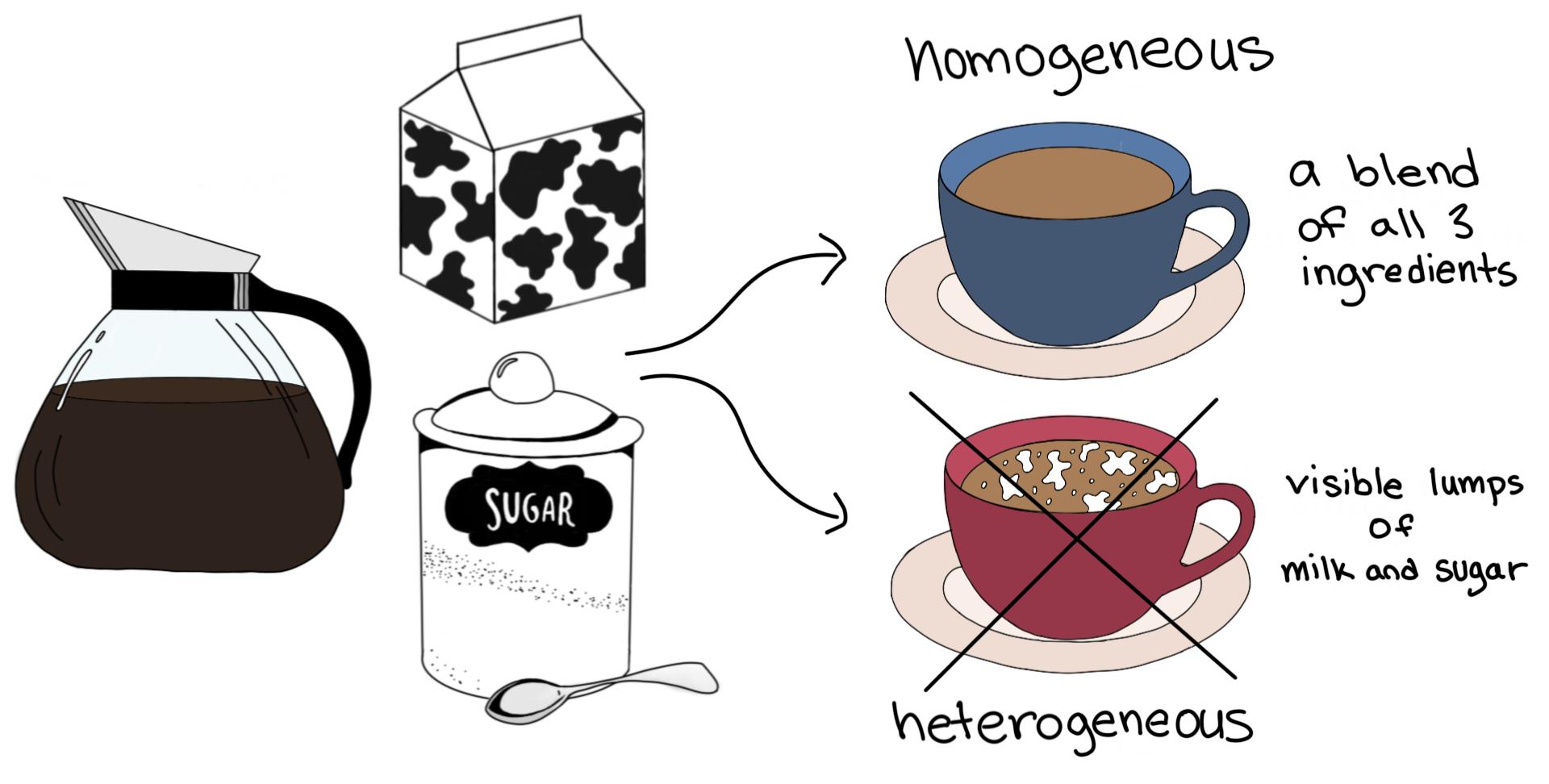
Homogeneous Heterogeneous Homogeneous Mixture Mixtures having a uniform composition all through the substance are called Homogeneous Mixtures For instance – a mixture of salt and water, a mixture of sugar and water, air, lemonade, soft drink water, and so on Here, a classic example is the mixture of salt in waterHomogeneous Mixtures Heterogeneous Mixtures centrifugation coagulation distillation evaporation filtration hand picking magnetic separation sieving winnowing sedimentation Mixture Homogenous Mixtures A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample Often it is easy to confuse a homogeneous mixture with a pure substance because they are both




A Z Of Classification Of Matter What Is The Classification Of Matter By Science Books Medium



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
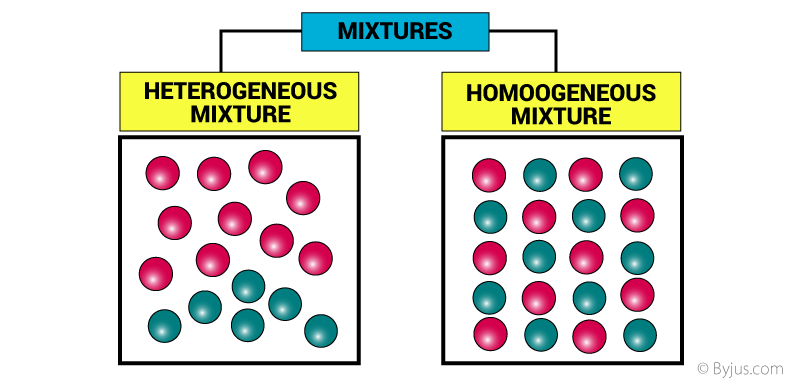



What Is A Mixture Definition Properties Examples Types With Videos




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




What Is A Homogeneous Mixture Definition And Examples
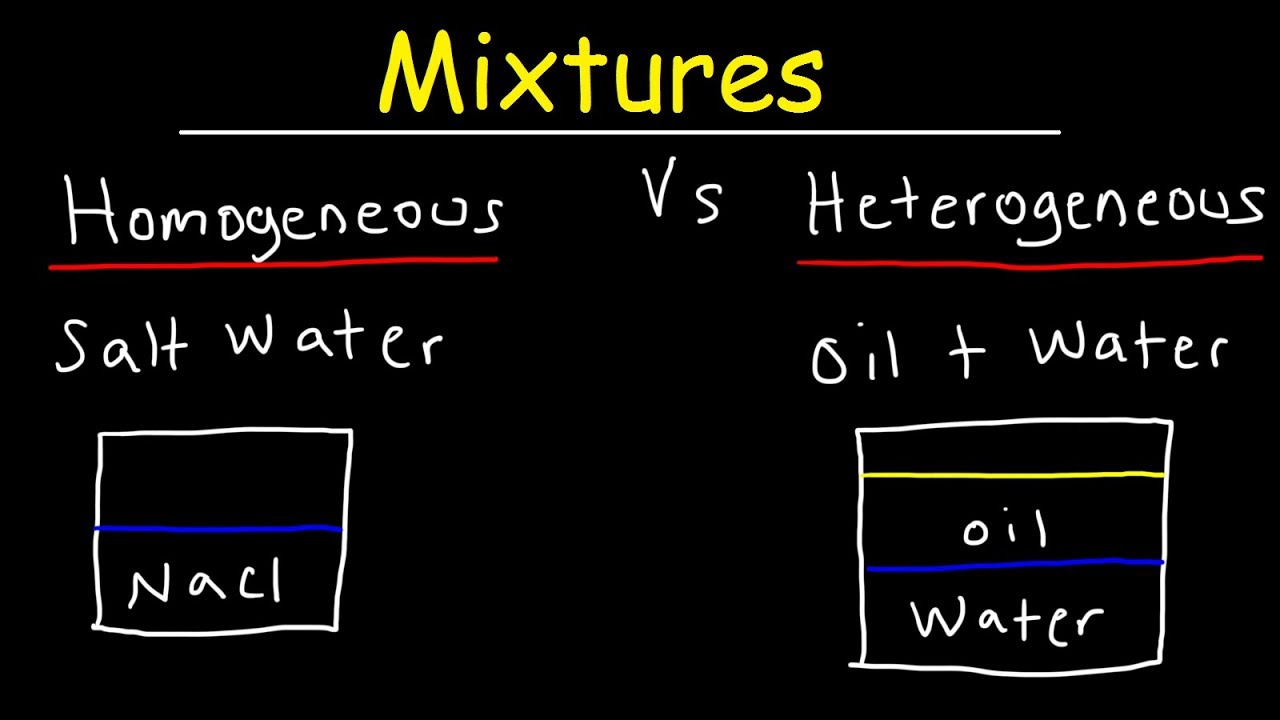



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




Homogeneous Mixture Definition Examples Tutors Com




Mixture Homogeneous And Heterogeneous Mixtures Ck 12 Foundation




Homogeneous Mixture Definition Examples Video Lesson Transcript Study Com




Heterogeneous Mixture Definition Science Trends
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples
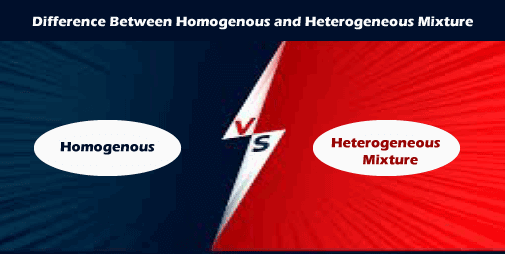



Difference Between Homogenous And Heterogeneous Mixture Javatpoint



Difference Between Homogeneous And Heterogeneous Welding




Heterogeneous Vs Homogeneous Definitions The Prefix Homo Indicate Sameness Homogeneous A Homogeneous Mixture Has The Same Uniform Appearance Throughout Ppt Download



3




10 Examples Of Mixtures



Homogeneous And Heterogeneous Mixture Nine Science
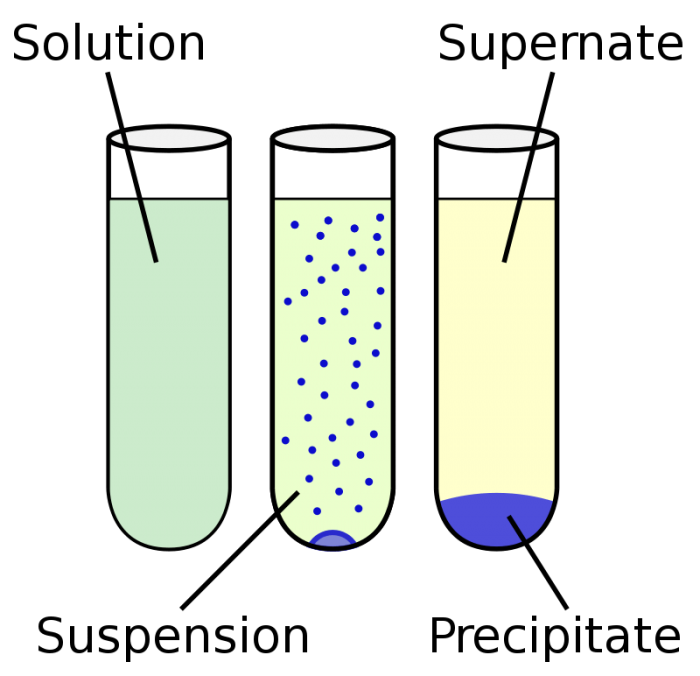



5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms
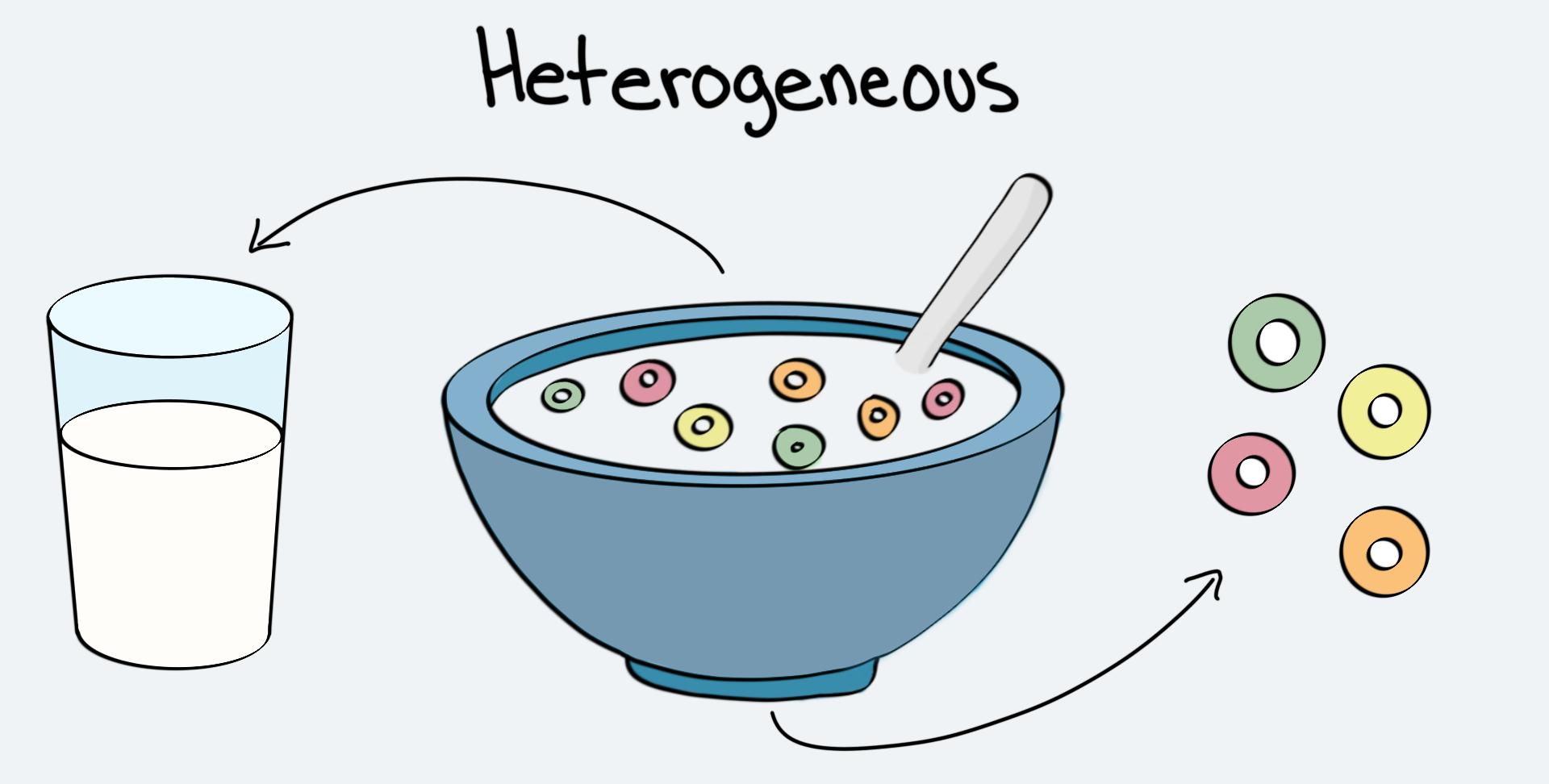



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Is Sugar A Homogeneous Or Heterogeneous Mixture Quora




Pure Substances And Mixtures Homogeneous Mixture Heterogeneous Mixture Elements Compounds And Mixtures




Organization Of Matter Scientific Terms Defined Pure Substance Contains Only One Type Of Particle Homogeneous Mixture Solution Made Up Of At Least Ppt Download
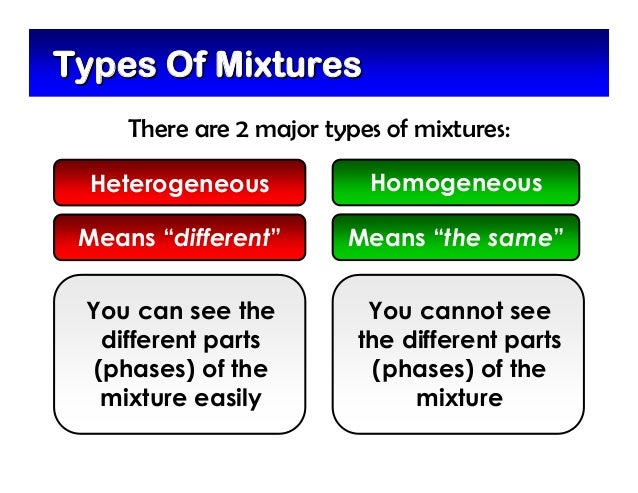



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com




Is Salty Water Homogeneous Or Heterogeneous




Ethiopia Learning Chemistry Grade 12 Page 7 In English
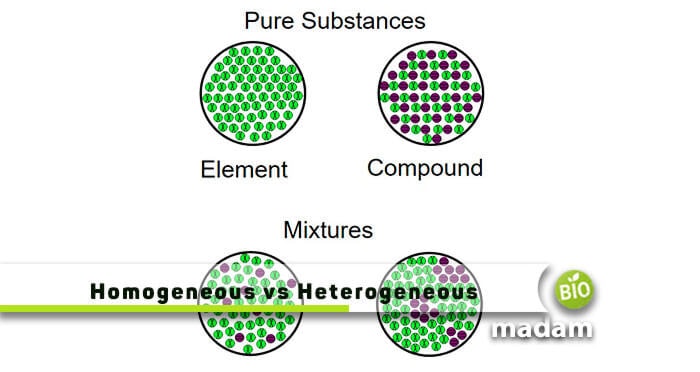



Difference Between Homogeneous Heterogeneous Mixtures Biomadam




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




Lesson Plan Homogenous And Heterogeneous Mixtures




Define A Heterogeneous Mixture And Give An Example Chegg Com




Homogeneous Mixture Definition




Neet Ug Solutions Definition Homogeneous And Heterogeneous Mixture Colloid And Suspension In Hindi Offered By Unacademy




Chemistry




Homogeneous Mixture Definition Examples Tutors Com



Mixtures 8th Grade Science Brogden
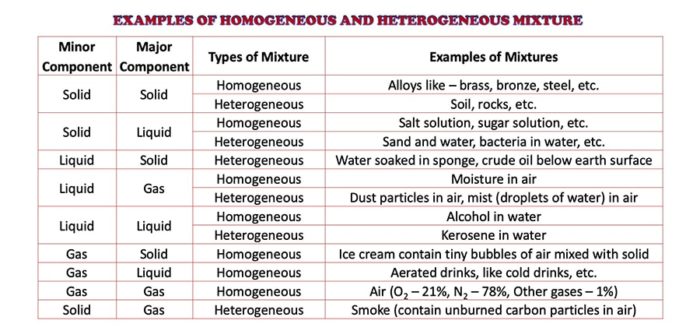



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Introduction And What Is A Mixture Types Classification Video Examples




Q1 Define Homogenous And Heterogeneous Mixture And Chegg Com




3 4 Classifying Matter According To Its Composition Chemistry Libretexts
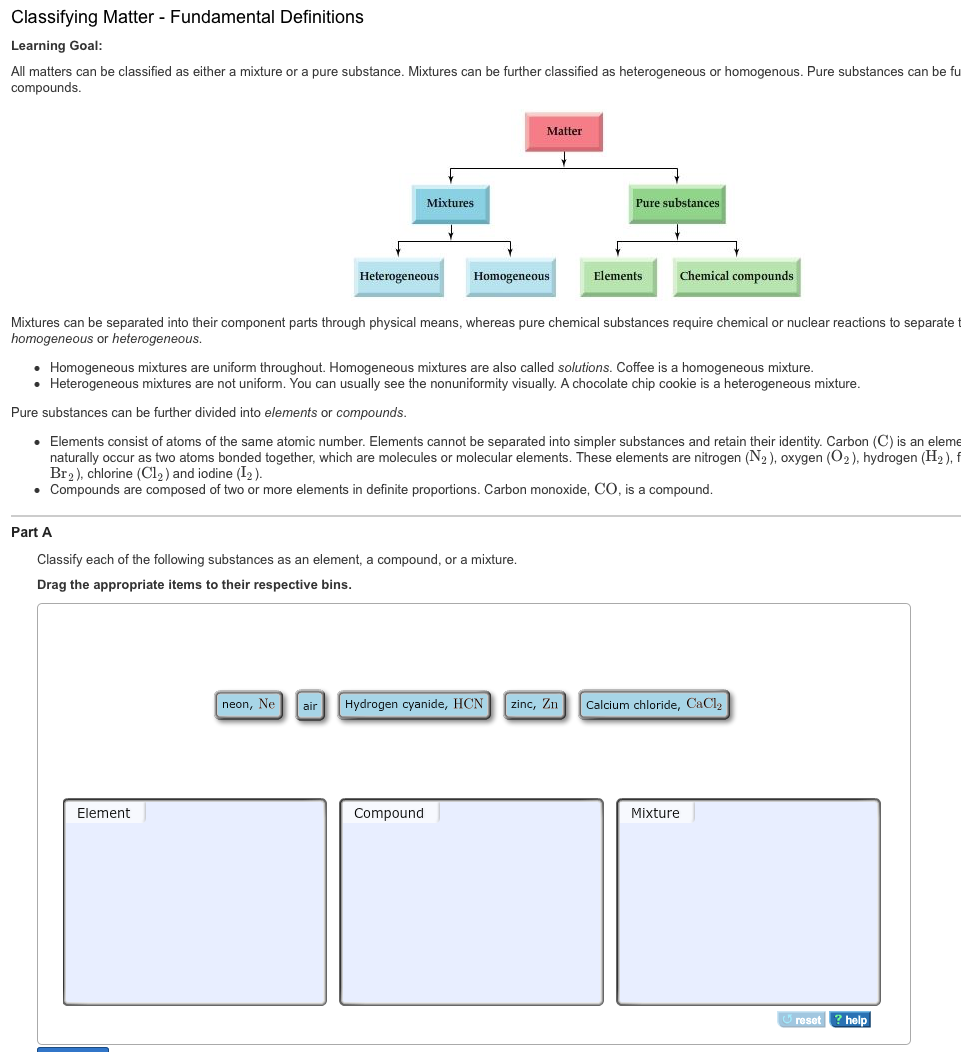



Classifying Matter Fundamental Definitions Chegg Com




Homogenous Definition And Examples Biology Online Dictionary




Homogenous Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool
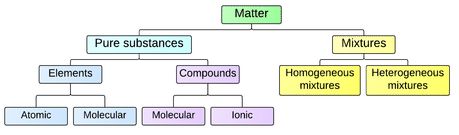



2 2 Matter Chemistry Libretexts



Types Of Catalysis




Learning Objectives Define The Following Terms Mixture Solution Solute Solvent Suspension Homogeneous Heterogeneous Identify A Mixture As Either Ppt Download




Difference Between Homogeneous And Heterogeneous Material Youtube
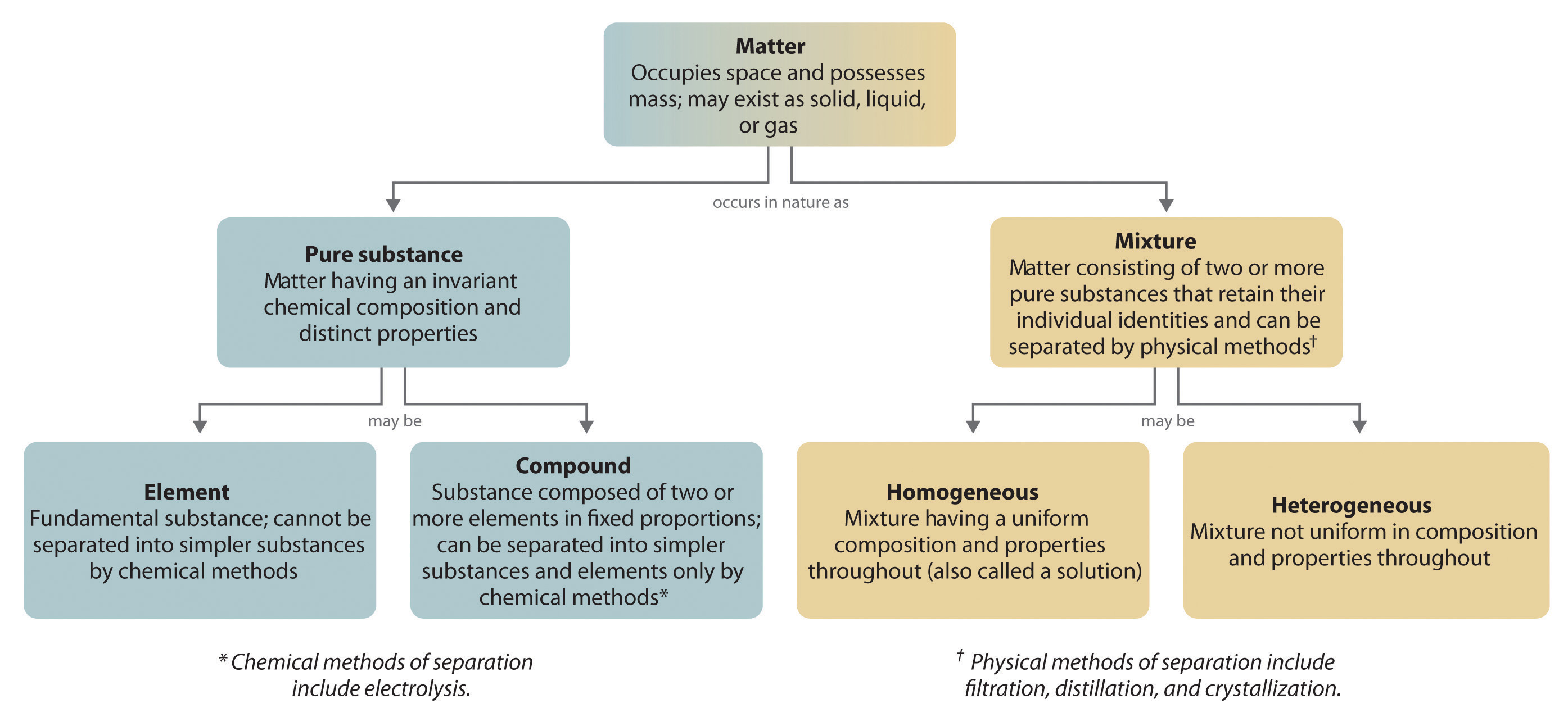



1 3 Classification Of Matter Chemistry Libretexts
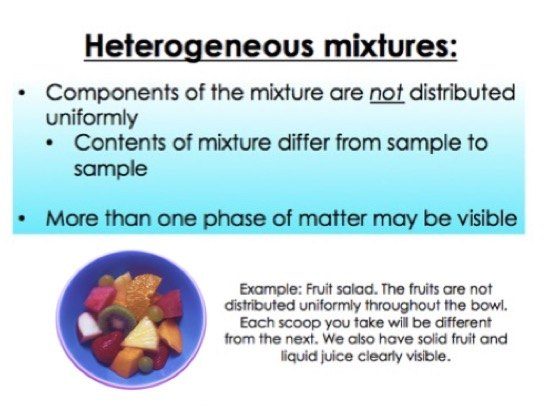



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



1




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Pin On Middle School Chemistry




Compound Vs Mixture Difference And Comparison Diffen




Homogenous Definition And Examples Biology Online Dictionary




Give Three Differences Between Homogeneous Mixture And Heterogeneous Mixture Brainly In




What Is A Heterogeneous Mixture Definition And Examples




Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Youtube




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
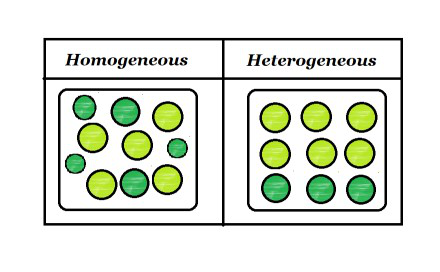



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



3




Solutions Unit 3 Solution It Is A Homogeneous Mixture That Is Formed When A Substance Is Dissolved In Another Substance Ppt Download
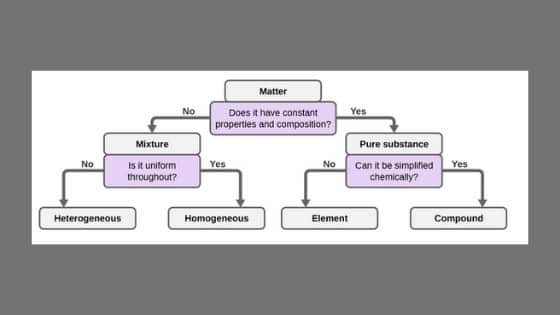



Homogeneous Mixture And Heterogeneous Mixture Ncert Books



Homogeneous And Heterogeneous Mixtures




2 3 Pt A Define A Homogeneous Mixture And Give Chegg Com
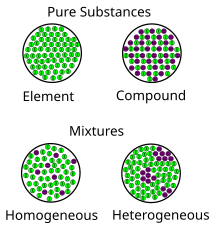



Mixture Wikipedia




What Is Suspension In Science Definition Types Examples Video Lesson Transcript Study Com




Homogeneous Vs Heterogeneous Mixture Youtube




2 1 Matter




Examples Of Heterogeneous Mixtures Types Made Simple




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry
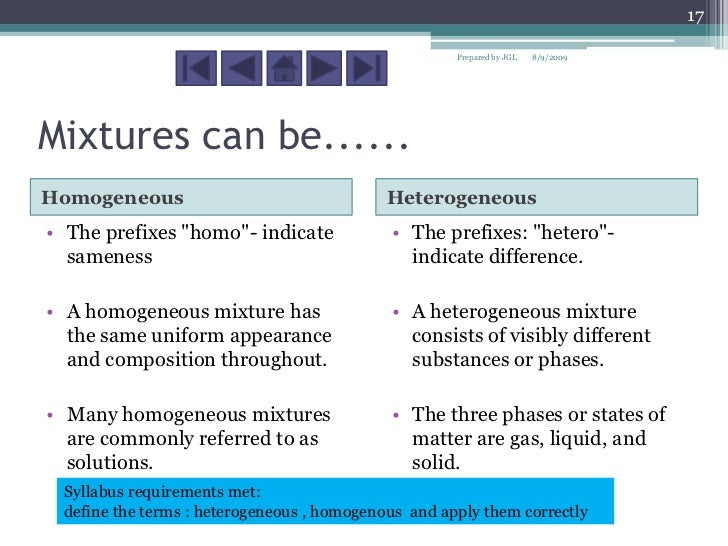



Mixtures And Their Separations



Is There Any Difference Between Homogeneous Mixture And Solution Here On Quora Previous Answers Are Vague About This While All My Textbooks And Google Sites Say They Are Exactly Same Quora


